Adenosine is a metabolic common pathway of rapid antidepressant action: the coffee paradox

The coffee paradox in adenosine-mediated antidepressant action. Depression (left) and coffee consumption (right) are both linked through adenosine signaling (center), creating a pharmacological paradox.
Your morning coffee might be blocking the same brain pathway that makes ketamine and electroshock therapy work for severe depression
Is this merely coincidence, or does it reveal something fundamental about why humans have gravitated toward caffeine consumption across cultures and millennia? A commentary published today in Brain Medicine by Drs. Julio Licinio and Ma-Li Wong explores this striking convergence and its profound implications for treating depression.
For over twenty years, psychiatrists have wielded two of their most powerful weapons against treatment-resistant depression without fully understanding why they worked. Ketamine, originally developed as an anesthetic and notorious as a party drug, could lift severe depression within hours. Electroconvulsive therapy, despite its controversial history and cognitive side effects, remained the most effective intervention when nothing else helped.
These treatments seemed to have nothing in common. Ketamine blocks NMDA receptors in the brain. ECT induces controlled seizures through electrical stimulation. Different mechanisms, different delivery methods, different side effect profiles. Yet both achieved something that traditional antidepressants could not: rapid, robust relief for patients who had tried everything else. The scientific community proposed various theories, but the unifying thread remained elusive.
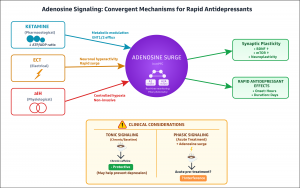
What finally solved this two-decade mystery? Professor Min-Min Luo and colleagues at the Chinese Institute for Brain Research have now revealed the answer. Their landmark study, published in Nature (10.1038/s41586-025-09755-9), identifies adenosine signaling as the convergent mechanism underlying both ketamine and ECT.
Using genetically encoded adenosine sensors, Luo's team monitored real-time adenosine dynamics in the brains of mice. When they administered ketamine, they observed rapid adenosine surges in key mood-regulatory regions including the medial prefrontal cortex and hippocampus. ECT produced similar adenosine increases, though with faster onset and decay.
The team then performed the critical experiments that established causation rather than mere correlation. When they genetically deleted adenosine A1 and A2A receptors, both ketamine and ECT lost their antidepressant effects entirely. The treatments no longer worked. Conversely, when they directly activated these same adenosine receptors, they replicated the antidepressant response without ketamine or ECT.
Luo's team went further, demonstrating that ketamine increases adenosine by modulating cellular metabolism rather than causing neuronal hyperactivity. They showed that ketamine directly impacts mitochondrial function, disrupting the intracellular ATP/ADP ratio and triggering adenosine efflux through equilibrative nucleoside transporters. Most remarkably, they identified a third intervention that works through the same pathway: acute intermittent hypoxia, controlled reductions in oxygen levels. This non-pharmacological approach produced antidepressant effects in mouse models that were entirely dependent on adenosine receptor activation.
But what does Luo's mechanistic discovery mean for the millions of people who drink coffee every day? Caffeine is an adenosine receptor antagonist. It blocks the very same A1 and A2A receptors that Luo showed are essential for rapid antidepressant action. Americans consume an average of 200 milligrams of caffeine daily, primarily from coffee and tea. Many consume far more. And patients showing up for ketamine infusions or ECT sessions routinely arrive having consumed their morning coffee.
"This is where rigorous mechanistic neuroscience collides with everyday clinical practice," explains Dr. Licinio. "We now have definitive evidence that adenosine receptor activation is not just helpful but essential for these treatments to work. Yet we have been completely overlooking the fact that our patients are chronically blocking these same receptors with caffeine. The implications are staggering."
How can coffee be both protective against depression and potentially interfere with depression treatment? The relationship between caffeine and depression is complex and seemingly contradictory. Large epidemiological studies consistently show that regular coffee drinkers have lower rates of depression compared to non-drinkers. A meta-analysis of over 300,000 participants found that coffee consumption was associated with a significant decrease in depression risk.
Yet Luo's work suggests that acute caffeine consumption might interfere with the very treatments we use for severe depression. The answer likely lies in distinguishing chronic effects from acute interference. The epidemiological protection that chronic coffee drinking confers against depression may represent an inadvertent form of adenosinergic modulation operating at population scale. Regular caffeine consumption might provide tonic benefit through complex adaptive changes in adenosine receptor expression and sensitivity.
But the same mechanism that provides long-term benefit might interfere with the phasic therapeutic surges that occur during acute treatment. When ketamine or ECT trigger a rapid adenosine spike, that signal needs to reach adenosine receptors to produce its antidepressant effect. If those receptors are occupied by caffeine, the signal cannot get through.
"Think of it like trying to charge your phone while someone else has their charger plugged into the outlet," offers Dr. Wong. "The power source is working, but it cannot reach your device. Ketamine and ECT are generating the adenosine signal, but if caffeine is blocking the receptors, that signal cannot produce its therapeutic effect."
What clinical questions does this discovery raise? Licinio and Wong's commentary identifies several urgent research priorities that emerge from Luo's mechanistic insights. First, do regular coffee drinkers show altered responses to ketamine or electroconvulsive therapy? Existing clinical trial data may already contain this information, buried in baseline demographic variables that no one thought to analyze systematically. A retrospective analysis of ketamine trials, stratifying patients by caffeine consumption, could provide immediate answers.
Second, does pre-treatment caffeine washout enhance therapeutic outcomes? This could be tested prospectively in a randomized trial where patients either continue their usual caffeine intake or abstain for a specified period before treatment. The mechanistic foundation is strong enough to justify such a study.
Third, are there individual differences in vulnerability to caffeine interference? Genetic variants in adenosine receptors or caffeine metabolism might predict who will experience the most interference. Pharmacogenomic approaches could identify patients who need to be most cautious about caffeine consumption around treatment times.
Fourth, can we develop dosing strategies that preserve the protective effects of chronic caffeine consumption while optimizing acute treatment responses? Perhaps patients could maintain their regular coffee intake but abstain for 24 hours before each ketamine session or ECT treatment. Finding this balance could maximize both chronic protection and acute therapeutic benefit.
Does adenosine signaling open doors to entirely new treatments? The implications of Luo's work extend far beyond the coffee question. By identifying adenosine as a tractable therapeutic target, his team has opened the door to entirely new treatment approaches.
Acute intermittent hypoxia represents one such possibility. Unlike ketamine, which carries risks of abuse and dissociative side effects, or ECT, which requires anesthesia and can cause cognitive impairment, intermittent hypoxia is non-invasive and well-tolerated. Luo's team showed that controlled cycles of brief oxygen reduction produce antidepressant effects in mice through adenosine-dependent mechanisms. Intermittent hypoxia has an established safety profile in humans and has been studied extensively for neuroprotection and respiratory training. Adapting these protocols for depression treatment could provide a scalable alternative to ketamine clinics and ECT suites.
The adenosine framework also suggests other possibilities. Could we develop drugs that enhance adenosine signaling more selectively than ketamine? Luo's team synthesized several ketamine derivatives with enhanced adenosine-releasing properties and reduced side effects. One compound, deschloroketamine, produced significant antidepressant effects at doses one-fifth those required for ketamine, with minimal locomotor activation.
Could we use adenosine dynamics as a biomarker to predict treatment response? Real-time monitoring of adenosine levels during ketamine infusion might identify patients who are generating inadequate adenosine surges, either due to metabolic differences or caffeine interference. Such patients might need dose adjustments or caffeine washout. Could we develop combination approaches that enhance adenosine signaling through multiple mechanisms? Perhaps pairing low-dose ketamine with intermittent hypoxia could produce synergistic effects, allowing lower doses of ketamine with fewer side effects.
What makes the adenosine mechanism so elegant? One of the most compelling aspects of Luo's work is the mechanistic detail it provides about how ketamine increases adenosine levels. Rather than proposing vague effects on neurotransmission, the team traces a clear path from drug administration to therapeutic outcome.
Ketamine directly impacts mitochondrial metabolism in neurons and glia. By incubating isolated brain mitochondria with radioactively labeled pyruvate, Luo's team showed that ketamine dose-dependently suppresses entry of this substrate into the tricarboxylic acid cycle. This metabolic brake decreases the intracellular ATP/ADP ratio. When cellular energy status drops, adenosine accumulates inside cells. This intracellular adenosine then exits through equilibrative nucleoside transporters, which maintain balance between intracellular and extracellular adenosine concentrations.
This metabolic mechanism has important implications. It suggests that ketamine is not just another psychiatric drug acting on neurotransmitter receptors, but rather a metabolic neuromodulator that harnesses the brain's own energy-sensing machinery to produce therapeutic effects. It also helps explain why ketamine works so quickly. Metabolic changes occur within minutes, much faster than the synaptic plasticity changes that were previously proposed as the primary mechanism.
How does this framework help us understand ECT? Electroconvulsive therapy has remained medicine's most effective treatment for severe depression despite decades of attempts to understand and improve it. Luo's work finally provides mechanistic clarity. ECT induces neuronal hyperactivity through electrical stimulation. This intense activity rapidly depletes cellular energy stores, triggering adenosine release as a protective mechanism against overexcitation. The adenosine surge serves dual purposes: it dampens excessive neuronal firing and simultaneously activates the therapeutic pathways that relieve depression.
This framework helps explain ECT's side effects. The widespread neuronal activation that generates therapeutic adenosine release also disrupts memory formation and can cause confusion. Understanding the adenosine mechanism might enable more targeted delivery of electrical stimulation to mood-regulatory regions while sparing areas critical for memory. It also suggests why ECT works when ketamine fails. Some patients do not respond adequately to ketamine, perhaps because the drug cannot generate sufficient adenosine in their particular neural circuitry.
Does adenosine provide a unified framework for understanding depression treatment? Licinio and Wong's commentary emphasizes how Luo's adenosine framework unifies seemingly disparate observations about depression treatment. Sleep deprivation, long known to have acute antidepressant effects, increases adenosine levels. Ketogenic diets, which some studies suggest may benefit mood, alter cellular metabolism in ways that could impact adenosine. Exercise, perhaps the most consistent lifestyle intervention for depression, increases adenosine signaling.
The framework even sheds light on why traditional antidepressants take weeks to work while ketamine acts within hours. Selective serotonin reuptake inhibitors and similar drugs gradually induce downstream changes that eventually affect adenosine signaling, but only after chronic administration. Ketamine directly triggers adenosine release, bypassing the slow cascade.
"What Luo has given us is not just an explanation for ketamine and ECT, but a lens through which we can reinterpret the entire landscape of depression therapeutics," notes Dr. Licinio. "Adenosine emerges as a final common pathway that many interventions eventually engage, whether pharmacological, electrical, metabolic, or behavioral."
What should happen next? The coffee paradox demands resolution through carefully designed clinical studies. The mechanistic foundation is solid. The clinical questions are clear. What remains is execution. Professional societies should issue guidance on caffeine consumption around ketamine and ECT treatments. Even in the absence of definitive trial data, the mechanistic rationale is strong enough to warrant precautionary recommendations.
Ketamine clinics should systematically collect data on patients' caffeine consumption and analyze whether it correlates with treatment response. This low-cost approach could quickly generate preliminary evidence. Research funding agencies should prioritize studies examining the adenosine-caffeine interaction in depression treatment. The convergence of Luo's mechanistic insights with the widespread use of both caffeine and rapid-acting antidepressants creates an unusually clear opportunity for translational research.
Drug developers should pursue adenosine-targeted approaches with renewed urgency. Luo's work provides validated targets and proof-of-concept data. The derivatives his team synthesized demonstrate that improved therapeutic windows are achievable.
"We are at one of those rare moments where basic neuroscience, clinical observation, and everyday human behavior intersect in ways that could transform treatment," reflects Dr. Wong. "The world's most popular psychoactive drug may be interfering with our most effective rapid antidepressants. Understanding this intersection is not just scientifically fascinating, it is clinically urgent."
Luo's identification of adenosine as the pivotal mediator of rapid antidepressant action represents a landmark achievement in psychiatric neuroscience. By establishing causal necessity through genetic deletion studies, demonstrating sufficiency through receptor activation, and revealing mechanistic detail through metabolic tracing, his team has set a new standard for understanding how psychiatric treatments work.
Licinio and Wong's commentary translates that mechanistic clarity into clinical imperatives. The coffee paradox, that caffeine blocks the very receptors essential for our best depression treatments, exemplifies how fundamental discoveries reshape therapeutic strategy. The convergence of the world's most prevalent psychoactive drug with the mechanistic lynchpin of our most effective rapid antidepressants is unlikely to be accidental. Understanding this intersection may illuminate both the widespread appeal of caffeine and the optimization of adenosine-targeted therapeutics.
From mechanism to bedside, from coffee cups to ketamine clinics, the adenosine story is just beginning.
Sources: The Commentary in Brain Medicine titled "Adenosine as the Metabolic Common Path of Rapid Antidepressant Action: The Coffee Paradox," is freely available via Open Access, starting on 11 November 2025 in Brain Medicine at the following hyperlink: https://doi.org/10.61373/bm025c.0134.
The Nature study by Yue and colleagues led by Professor Min-Min Luo titled “Adenosine signalling drives antidepressant actions of ketamine and ECT," is available at: https://doi.org/10.1038/s41586-025-09755-9.
About Brain Medicine: Brain Medicine (ISSN: 2997-2639, online and 2997-2647, print) is a peer-reviewed medical research journal published by Genomic Press, New York. Brain Medicine is a new home for the cross-disciplinary pathway from innovation in fundamental neuroscience to translational initiatives in brain medicine. The journal's scope includes the underlying science, causes, outcomes, treatments, and societal impact of brain disorders, across all clinical disciplines and their interface.
Visit the Genomic Press Virtual Library: https://issues.genomicpress.com/bookcase/gtvov/
The Genomic Press website is at: https://genomicpress.com/
Ma-Li Wong
Genomic Press
maliwong7@gmail.com
Visit us on social media:
LinkedIn
Bluesky
Instagram
Facebook
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.